UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
(Exact name of registrant as specified in its charter)
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
| |
||
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code:
Not applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered | ||
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
| Item 8.01 | Other Events. |
On October 14, 2020, Arvinas, Inc. (the “Company”) will present information at the 3rd Targeted Protein Degradation Summit regarding the progress of its pipeline and details of its PROTAC Discovery Engine, including introducing five discovery programs in its pipeline of over twenty programs for the following protein targets and indications: BCL6 for B-cell malignancies; KRAS for non-small-cell lung carcinoma, colorectal cancer and pancreatic cancer; Myc for solid malignancies; HPK1 for solid malignancies; and mutant huntingtin (mHTT) for Huntington’s disease. The Company will also provide details of anticipated milestones for its current clinical programs: ARV-110 for the treatment of men with metastatic castrate-resistant prostate cancer; and ARV-471 for the treatment of patients with locally advanced or metastatic ER+/HER2- breast cancer. As previously disclosed, the Company expects to provide, through Company-driven communications or events, an update from its Phase 1/2 trial for ARV-110 and interim data from its Phase 1/2 trial for ARV-471 during the fourth quarter of 2020.
The portion of the presentation regarding the Company’s pipeline updates and anticipated milestones is attached hereto as Exhibit 99.1 and is incorporated by reference herein.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits
| Exhibit |
Description | |
| 99.1 | Excerpts from Company Presentation, dated October 14, 2020. | |
| 104 | Cover Page Interactive Data File (formatted as Inline XBRL). | |
Forward-Looking Statements
This Current Report on Form 8-K, including the document filed as Exhibit 99.1 hereto, contains forward-looking statements that involve substantial risks and uncertainties, including statements regarding the development and regulatory status of the Company’s product candidates, such as statements with respect to the Company’s lead product candidates, ARV-110, ARV-471 and ARV-766 and other candidates in the Company’s pipeline, and the timing of clinical trials and data from those trials and plans for registration for the Company’s product candidates, and the Company’s development programs that may lead to the Company’s development of additional product candidates, the potential utility of the Company’s technology and therapeutic potential of the Company’s product candidates and the potential commercialization of any of the Company’s product candidates. All statements, other than statements of historical facts, contained in this Current Report on Form 8-K, including statements regarding the Company’s strategy, future operations, prospects, plans and objectives of management, are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words.
The Company may not actually achieve the plans, intentions or expectations disclosed in its forward-looking statements, and you should not place undue reliance on such forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements the Company makes as a result of various risks and uncertainties, including but not limited to: whether the Company will be able to successfully conduct Phase 1/2 clinical trials for ARV-110 and ARV-471, complete its clinical trials for its product candidates, and receive results from its clinical trials on the Company’s expected timelines, or at all, whether the Company’s cash resources will be sufficient to fund its foreseeable and unforeseeable operating expenses and capital expenditure requirements, the Company’s expected timeline and other important factors discussed in the “Risk Factors” sections contained in the Company’s quarterly and annual reports on file with the Securities and Exchange Commission. The forward-looking statements contained in this Current Report on Form 8-K reflect the Company’s current views with respect to future events, and the Company assumes no obligation to update any forward-looking statements except as required by applicable law. These forward-looking statements should not be relied upon as representing the Company’s views as of any date subsequent to the date of this Current Report on Form 8-K.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| ARVINAS, INC. | ||||||
| Date: October 14, 2020 | By: | /s/ Sean Cassidy | ||||
| Sean Cassidy Chief Financial Officer | ||||||

1 Today: Introducing five targets for which PROTAC® protein degraders have high potential to differentiate from other drug modalities KRAS Oncogenic cell growth regulator BCL6 Transcription factor implicated in B cell lymphomas Myc Oncogenic transcription factor driving tumor cell proliferation HPK1 Suppressor of T cell activation; immuno-oncology target mHTT Key target for Huntington’s disease Target Differential Biology Based on the Tenets of PROTAC® Degraders Target scaffolding function of BCL6 Target “undruggable” KRAS mutants (e.g., G12V, G12D) Directly degrade “undruggable” Myc vs. other indirect approaches Address potential scaffolding function Selectively degrade mutant huntingtin (mHTT) protein Exhibit 99.1

Most B cell lymphomas are dependent on constitutive or deregulated expression of BCL6, a transcriptional repressor of: Cell cycle checkpoints Terminal differentiation Apoptosis DNA damage response PROTAC® degradation would address the scaffolding function of BCL6 2 Arvinas’ BCL6 program is aiming for an oral, best-in-class targeted therapy for B-cell malignancies Optimizing in vivo tumor growth inhibition activity and selecting a candidate to take forward with anticipated IND in 2022 After oral dosing, PROTAC® X achieved >95% degradation of BCL6 in vivo Farage DLBCL xenograft model BCL6 Vehicle PROTAC® X 1 2 3 4 5 6 7 8 1 2 3 4 5 6 7 8 Tumor BCL6 GAPDH

3 We are taking a comprehensive approach to degrading KRAS KRAS is the most frequently mutated gene in human cancer and is a classic “undruggable” target due to its lack of deep “pockets” We are creating pan-KRAS mutant, in addition to mutant-specific (e.g., G12D and G12V), degraders As a proof of concept, we have successfully developed in vivo active KRAS G12C-specific PROTAC® degraders MiaPaCa-2 xenograft model Vehicle PROTAC® Y Leveraging learnings from KRAS G12C development to accelerate other KRAS degraders’ development with anticipated IND in 2023 Six hours after a single dose, PROTAC® Y degraded >80% of KRAS G12C in vivo KRAS Tumor
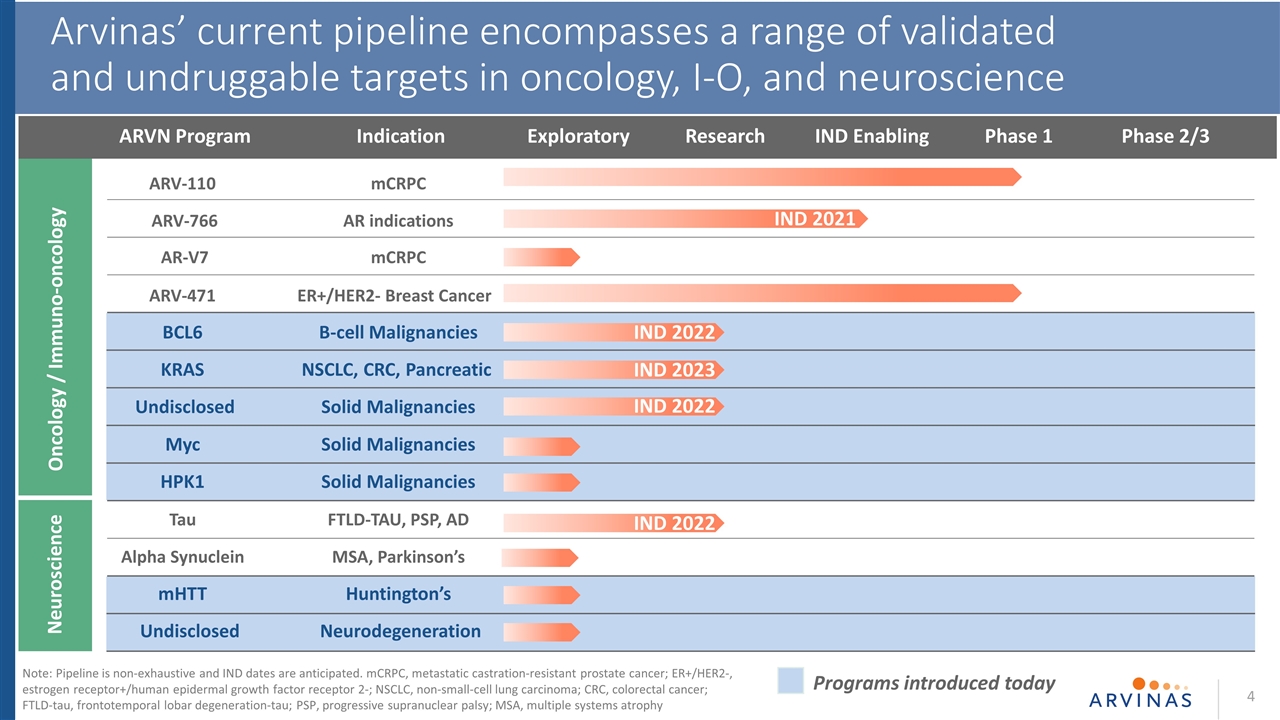
Neuroscience Oncology / Immuno-oncology ARVN Program Research IND Enabling Phase 1 Phase 2/3 Exploratory 4 Arvinas’ current pipeline encompasses a range of validated and undruggable targets in oncology, I-O, and neuroscience Indication IND 2021 IND 2022 IND 2023 IND 2022 Programs introduced today IND 2022 ARV-110mCRPC ARV-766AR indications AR-V7mCRPC ARV-471ER+/HER2- Breast Cancer BCL6B-cell Malignancies KRASNSCLC, CRC, Pancreatic UndisclosedSolid Malignancies MycSolid Malignancies HPK1 Solid Malignancies TauFTLD-TAU, PSP, AD Alpha Synuclein MSA, Parkinson’s mHTTHuntington’s Undisclosed Neurodegeneration Note: Pipeline is non-exhaustive and IND dates are anticipated. mCRPC, metastatic castration-resistant prostate cancer; ER+/HER2-, estrogen receptor+/human epidermal growth factor receptor 2-; NSCLC, non-small-cell lung carcinoma; CRC, colorectal cancer; FTLD-tau, frontotemporal lobar degeneration-tau; PSP, progressive supranuclear palsy; MSA, multiple systems atrophy

Program update Initiation of Phase 2 Completed Phase 1 data Phase 2 interim data Initiation of combination study Full Phase 2 data Combination study data ARV-110 (mCRPC) Interim Phase 1 data Initiation of combination study with CDK4/6i Completed Phase 1 data Initiation of Phase 2 CDK4/6i combination study data Interim Phase 2 data ARV-471 (ER+/HER2- breast cancer) ARV-766 BCL6 Undisclosed (oncology) Tau INDs Initiate Phase 1 Phase 1 data Initiate Phase 2 ARV-766 (AR PROTAC®) Over the next two years, we anticipate a rapid pace of milestones 2021 2020 Q4 2022 Note: mCRPC, metastatic castration-resistant prostate cancer; ER+/HER2-, estrogen receptor+/human epidermal growth factor receptor 2-

Arvinas 2024 Vision: Ascending to new heights in bringing the benefits of PROTAC® degraders to patients Built Arvinas’ Foundation as a Pioneer in Protein Degradation Proved the Concept of Our PROTAC® Discovery Engine 2013-2018 2019-2020 2024 Vision Integrated biotech poised for launch First PROTAC® degraders proven to benefit patients in registrational studies Sustainably nominating ≥1 clinical candidate per year Our PROTAC® Discovery Engine delivering candidates with tissue- and disease-specific degradation Completing build-out of the resources and capabilities to bring PROTAC® therapeutics to market
